What are electrolytes and how do they work? And do plants crave them?
Let’s start with…
Ions
An ion is an electrically charged particle, often an atom with a “missing” or “extra” electron. Molecules can be held together by ionic bonds in which oppositely charged atoms are attracted to each other by electrostatic forces. A common example of a substance with an ionic bond is table salt— sodium chloride or NaCl. The positively charged ion is called a cation and the negatively charged ion is called an anion. In table salt, Na+ is the cation and Cl- is the anion.
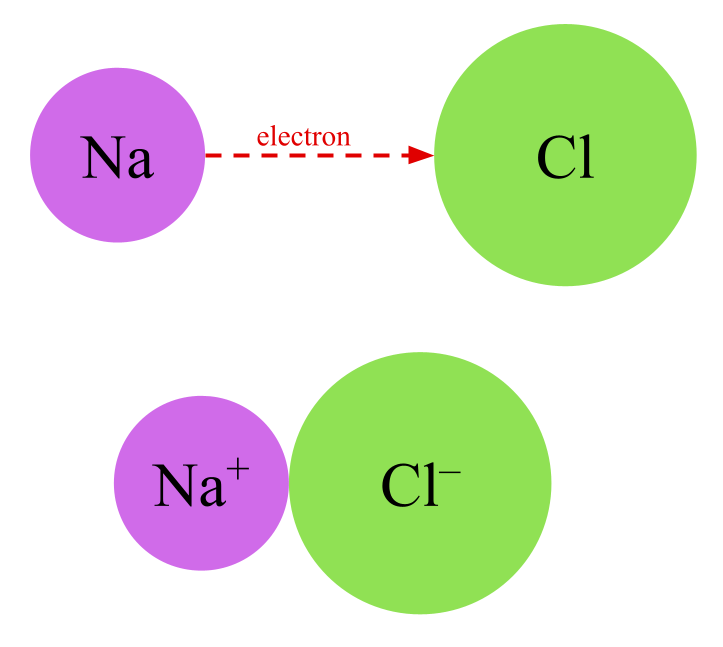
Other ionic compounds also form chemicals called salts, including potassium chloride, sodium fluoride, magnesium sulfate, silver nitrate, lithium cyanide, and so on.
When ions move, they create an electric current. This is different from a current created by the motion of electrons. In fact, an electric current can be created by the motion of positive ions. In the human body, this is how electrical signals are transmitted. Since the body does not contain conductive wires, it uses ion channels instead. This is why, for example, humans require potassium in our diet.
Electrolytes
An electrolyte is essentially an electrically neutral solution made up of electrically charged ions. It can conduct an electric current through the movement of ions. The most commonly known electrolyte is saltwater. Water does not conduct electricity well on its own, but water with dissolved minerals is very conductive.
Water is an important solvent because it is a polar molecule: one side is slightly electrically positive and the other side is slightly electrically negative. When a molecule like sodium chloride is in water, the two ions become separated and stick to the water molecules. The amount of available water molecules to stick ions to is limited, which is why if you keep adding salt to water it will eventually become saturated and stop dissolving. When water is cold, the molecules have a greater tendency to stick to one another, meaning there are few available molecules to stick ions to. When water is hot, the kinetic energy of its molecules prevent them from sticking together as much, making more molecules available. This is why more salt can be dissolved in warm water than in cold water (and warm water dissolves salt faster).
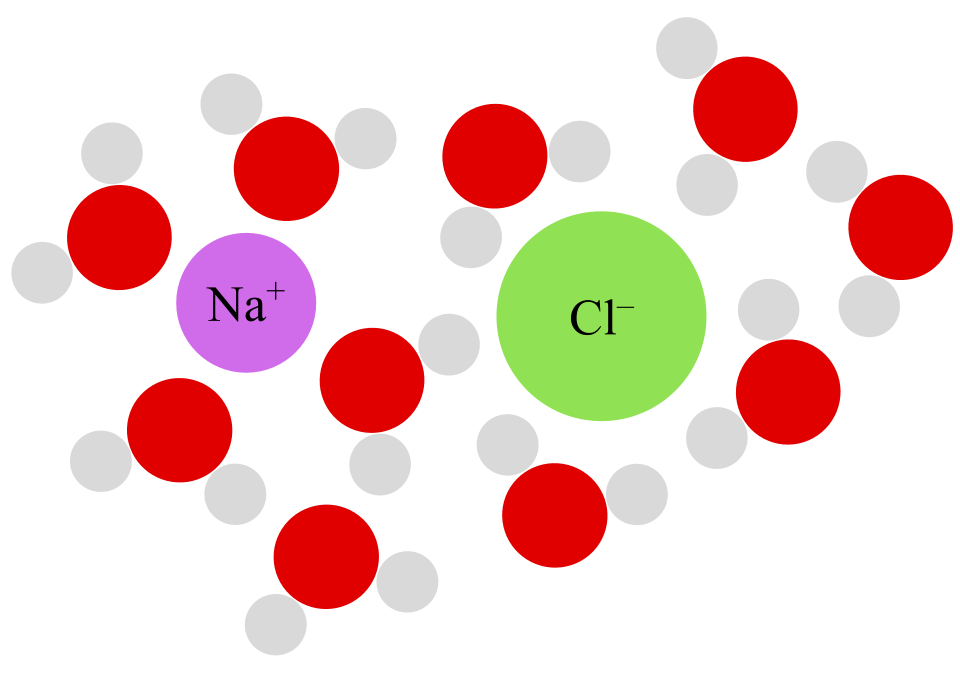
In short, consuming electrolytes is how we get dietary ions. Necessary ions for human life, particularly for the nervous system, include sodium, potassium, magnesium, calcium, chloride, phosphates, and bicarbonates. Because sweat contains a lot of sodium and chloride, sports drinks with electrolytes typically focus on replenishing these.
“Hydration multipliers”
The saturation of dissolved ions can also control the flow of water. This is thanks to osmosis, the passage of water through a semipermeable surface. Specifically, if two electrolyte solutions are separated by a membrane that allows only water to pass through, then water will always flow from the more saturated side to the less saturated side until the two sides have the same saturation level (when they are isotonic).
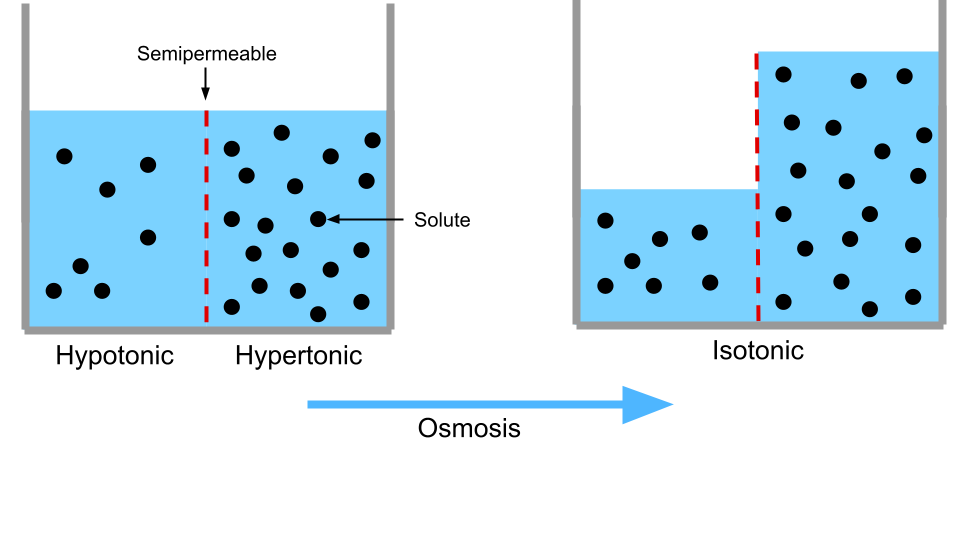
When drinking plain water, there is a slight issue because it is less saturated with ions than your bodily fluids. This has two effects: first, drinking a lot of plain water is inefficient for hydration because the body has trouble providing it with enough ions to be isotonic with your fluids. Excess water will be eliminated by the kidneys in order to maintain homeostasis. This is why “isotonic” beverages advertise being more hydrating than plain water— counterintuitively, having the dissolved salt in there makes the water more available to your body. Second, drinking excessive amounts of plain water will naturally result in ions being absorbed into the water from your body, essentially flushing them out. This is why drinking very large quantities of water without food is dangerous and can cause “water poisoning.”
Of course, if you have a diet that is high in salty foods, then this is not a concern. Likewise, eating too much salt without enough water is dehydrating and can cause different problems as a result of having too high saturation of ions in the body. Fortunately, the digestive system and particularly the kidneys are very good at adapting to varying levels of water and salt intake, and absorbing or eliminating water as needed.
What about plants?
Like animals, plants need electrolytes for their cells to function. They normally get their necessary ions from soil and water. Many plant fertilizers contain these ionic compounds, including potassium phosphate and potassium chloride among others. In general, there are three sets of three elements that plants need most, which can be summarized as carbohydrate components, primary nutrients, and secondary nutrients. Carbohydrate components are carbon, hydrogen, and oxygen. These are the basis for the organic molecules that make up the plant’s physical structure and are involved in respiration and so on, and mostly come from air and water. Primary nutrients are nitrogen, phosphorus, and potassium, often denoted NPK. Secondary nutrients are calcium, magnesium, and sulfur. These primary and secondary nutrients are the ionic building blocks of compounds that perform specialized functions. (Note that plants require trace amounts of certain other elements as well.)

So while humans require large quantities of sodium and chloride, plants do not. As a result, sports drinks or electrolyte beverages are not suitable for plants, and will tend to dry them out. In short, plants do crave electrolytes, but not the same ones humans crave. It is recommended that you test your soil’s chemical composition before adding fertilizer, as excess salts and nitrogen can cause fertilizer burn.
